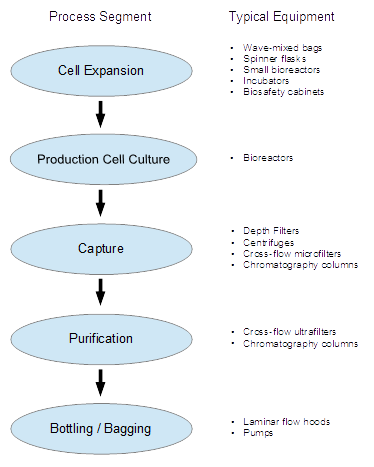
Schematic showing the general process flow for a typical biopharmaceutical process and the typical equipment involved in each area. Other areas (e.g., cell bank preparation, media preparation and buffer preparation) feed into these.
Biopharmaceutical manufacturing uses genetically engineered cells to grow product.

Any chemical production involves a series of major process steps called "unit operations". The specific unit operations are what make one industry different from another. The unit operations in biopharmaceutical manufacturing almost always include:
As with any pharmaceutical manufacturing, all of the work falls under the requirements of GMP (Good Manufacturing Practices).
The equipment used for these process is made almost entirely of stainless steel, with extremely smooth surfaces to improve cleanability.
The water used in the processes is highly purified. The specific processes used to purify the water depends on the quality and quantity used at a particular facility. A typical facility will make "purified water" and "water for injection" (WFI). WFI is one of the most pure grades of water on the planet.
Page updated 7/8/17 |
|